Introduction
Mim8 is in clinical development as a novel, next generation FVIII mimetic for subcutaneous prophylactic treatment for people with Hemophilia A (HA) with and without inhibitors. Mim8 is a fully human bispecific antibody bridging FIXa and FX. Mim8 showed approximately 15-fold higher potency compared to a sequence identical analogue (SIA) of the FVIII mimetic emicizumab (Hemlibra®) in thrombin generation assays (TGAs) and in vivo in mouse bleeding models (Kjellev et al., 2019; Ley et al. 2020). Mim8 binds to cynomolgus monkey and human FIX and FX with comparable affinities, and the pharmacological activity of Mim8 in cynomolgus monkeys was demonstrated both in vitro in TGA and in vivo in a cynomolgus bleeding model (Ley et al., 2020). The present study examined the safety and ex vivo pharmacodynamic effects of Mim8 in cynomolgus monkeys after weekly dosing for 26 weeks.
Material and Methods
Sexually mature cynomolgus monkeys were dosed subcutaneously (SC) with Mim8 at dose-levels of 0, 0.3, 1 or 3 mg/kg/week (4 males and 4 females at each dose-level) for 26 weeks. These dose-levels were chosen based on a previous 13-week safety study showing histopathological changes consistent with exaggerated pharmacology in a few animals dosed ≥6 mg/kg/week. During the study, the animals were monitored clinically and blood was sampled for measurement of standard clinical pathology, Mim8 exposure, anti-drug antibodies (ADA). The pharmacodynamic activity was assessed by ex vivo TGA in plasma under antibody-induced HA-like conditions and measurement of activated partial thromboplastin time (aPTT). After 26 weeks of exposure, the animals were euthanised, followed by macroscopic and microscopic pathological examination on all major organ systems.
Results
Weekly dosing with Mim8 at dose-levels up to 3 mg/kg/week for 26 weeks was well tolerated and the macroscopic and microscopic pathological examinations revealed no thrombi or other signs of excessive coagulation activation. In accordance, no significant changes in platelet counts, fibrinogen concentration or prothrombin time were observed.
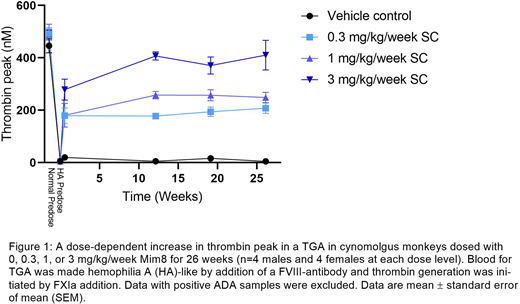
A pharmacodynamic effect was observed in dosed animals as a dose-dependent increase in ex vivo thrombin generation (TG; Figure 1) and shortening of aPTT.
The steady state exposure increased with increasing dose level in a dose-proportional manner, reaching concentrations at least 10-fold above the expected clinical concentrations. However, as expected when dosing a human antibody to monkeys, ADAs developed in approximately half of the animals at all dose levels, leading to lack of exposure and reversal of the aPTT and TG towards the predose levels.
Conclusion
In conclusion, administration of up to 3 mg/kg/week Mim8 to cynomolgus monkeys for 26 weeks showed relevant pharmacodynamic effect and was well tolerated with no observation of thrombi or other signs of excessive coagulation activation, providing a favourable safety ratio to expected clinical exposure levels. Mim8 is currently being evaluated in a clinical Phase 1/2 trial.
References
Kjellev SL et al. (2019) Mim8 - a next-generation FVIII mimetic bi-Specific antibody - potently restores the hemostatic capacity in hemophilia a settings in vitro and in vivo. Blood, 134 (Supplement 1): 96.
Ley CD et al. (2020) Improved effect of Mim8, a next-generation FVIII mimetic, translates from human in vitro to humanized mouse and cynomolgus models. European Association for Haemophilia and Allied Disorders (EAHAD 2020). Haemophilia, 26 (S2), P028.
Kjalke:Novo Nordisk A/S: Current Employment, Current equity holder in publicly-traded company.
Author notes
Asterisk with author names denotes non-ASH members.